molar mass of nacl|formula mass of nacl : Clark Enter the chemical formula of a compound and get its molar mass in g/mol. Learn how to calculate molar mass using the general formula and examples of NaCl, . WEBJoin millions of players in the world's first cross-platform hunting action RPG. Play free now. PS4 / PS5 Xbox Nintendo Switch PC. Dauntless is a free-to-play co-op action RPG available for Nintendo Switch, PS4, PS5, Xbox, and PC on the Epic Games store, with true cross-play and cross-progression.
0 · relative formula mass of nacl
1 · molar mass of nh3
2 · molar mass of na2so4
3 · molar mass of na2co3
4 · molar mass of c2h5oh
5 · gram formula mass of nacl
6 · formula mass of nacl
7 · More
8 · 1 mole of nacl
webGameTwist je platformou pro kasinové hry, jež si v herních záležitostech stojí za moderností. Je jedno, zdali hrajete raději na desktopu a velké obrazovce nebo dáváte přednost mobilní zábavě v kasinu na svém mobilu: Očekává vás vynikající kvalita her, jež můžete v mnoha případech hrát dokonce zdarma , a tak propůjčíte mnoha všedním .
molar mass of nacl*******Molar Mass of a Compound. One mole of any compound will have a mass that is numerically equal to its molecular mass or formula mass and expressed in units of .Learn how to calculate the molar mass of NaCl using the formula and the atomic masses of Na and Cl. The answer is 58.44 g mol - 1.
Enter the chemical formula of a compound and get its molar mass in g/mol. Learn how to calculate molar mass using the general formula and examples of NaCl, .
NaCl (Sodium chloride) molar mass. Enter a chemical formula to calculate its molar mass and elemental composition: Molar mass of NaCl (Sodium chloride) is 58.4428 g/mol. . Learn the definition and formula of molar mass, and how to calculate it for elements and compounds. Find the molar mass of NaCl and compare it with molecular .molar mass of nacl Learn how to calculate the molar mass of a substance by summing the molar masses of its component atoms, and how to use it to convert between mass .There are 4 easy steps to find the molar mass of NaCl (S) based on its chemical formula. 1. Count The Number of Each Atom. The first step to finding the molar mass is to count .Molar mass of NaCl = 58.44277 g/mol. This compound is also known as Sodium Chloride. Convert grams NaCl to moles. or. moles NaCl to grams. Molecular weight calculation: . The molar mass of O 2 is 16.00 x 2 = 32.00 g/mol. Example #2: Find the Molar Mass of NaCl. Apply the same step and find the molar mass of table salt or NaCl. The formula is NaCl. Atomic mass of Na = 22.99; atomic mass of Cl = 35.45. Molar mass of NaCl = 22.99 + 35.45 g/mol. Example #3: Find the Molar Mass of CO 2. Find the .Total Mass. 58.439. The molar mass of NaCl (sodium chloride) is: 58.439 grams/mol. See also our theoretical yield calculator for chemical reactions (probably your next stop to finish the problem set).If we have a chemical compound like NaCl, the molar mass will be equal to the molar mass of one atom of sodium plus the molar mass of one atom of chlorine. If we write this as a calculation, it looks like this: (1 atom x 23 grams/mole Na) + (1 atom x 35.5 grams/mole Cl) = 58.5 grams/mole NaCl .We can use the rearranged molarity equation to calculate the moles of NaCl needed for the specified concentration and volume: mol NaCl = [ NaCl] × L of solution = 0.800 mol L × 0.250 L = 0.200 mol NaCl. We can then use the molecular weight of sodium chloride, 58.44 g mol , to convert from moles to grams of NaCl :
Molar mass of (NaCl) = 58.44277 g/mol. Convert grams (NaCl) to moles. or. moles (NaCl) to grams. Molecular weight calculation: (22.98977 + 35.453) Percent composition by element. Element: Sodium Symbol: Na Atomic Mass: 22.989770 . Finding molar mass starts with units of grams per mole (g/mol). When calculating molecular weight of a .Sodium chloride, also known as salt, common salt, table salt or halite, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. Sodium chloride is the primary salt in seawater and in the extracellular fluid of many multicellular organisms.formula mass of naclSodium chloride, also known as salt, common salt, table salt or halite, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. Sodium chloride is the primary salt in seawater and in the extracellular fluid of many multicellular organisms. Let us start by calculating the formula mass of sodium chloride (NaCl). This formula mass is the sum of the atomic masses of one sodium atom and one chlorine atom, which we find from the periodic table; here, we use the masses to two decimal places: Na: 22.99 amu. Cl: +35.34 amu. Total: 58.44 amu.Molar mass of NaCl = 58.44277 g/mol. Convert grams Sodium Chloride to moles. or. moles Sodium Chloride to grams. Molecular weight calculation: 22.989770 + 35.453. Percent composition by element. Element: Sodium Symbol: Na Atomic Mass: 22.989770 # of Atoms: 1 Mass Percent: 39.337%. Element: ChlorineFinally, add together the total mass of each element to get the molar mass of NaCl(S): 22.98976928 g/mol + 35.453 g/mol + 32.065 g/mol = 90.50776928 g/mol. 5. Find Mole Fraction. To find the mole fraction and percentage of each element in NaCl(S), divide each total from step 3 by the total molar mass found in step 4:Calculando a massa molar passo a passo; Primeiro, calcule o número de cada átomo em NaCl: Na: 1, Cl: 1. Em seguida, pesquise os pesos atômicos para cada elemento da tabela periódica: Na: 22.98976928, Cl: 35.453 Agora, calcule a soma dos produtos do número de átomos pelo peso atômico: Massa molar (NaCl) = ∑ Count i * Weight i =Molar concentration, also known as molarity, and can be denoted by the unit M, molar. To prepare 1 L of 0.5 M sodium chloride solution, then, as per the formula, use 29.22 g of sodium chloride (0.5 mol/L * 1L * 58.44 g/mol = 29.22 g). The mass molarity calculator tool calculates the mass of compound required to achieve a specific molar .
Molar mass: 58,44277 g/mol. The number of atoms: 2 - Na, Cl. Melting point: 800.8 ° C. Boiling point: 1465 ° C. Solubility: in water, in methanol, in ammonia. Sodium chloride is the sodium salt of hydrochloric acid. It is included in table salt as the main component. It is present in large quantities in seawater and is a precondition for its . The molar mass of Chlorine is 35.45 g/mol. [2] Now, to calculate the molar mass of NaCl, you just have to add the molar mass of all the individual atoms that are present in NaCl. You can see that in NaCl, there is 1 Sodium atom and 1 Chlorine atom. So, Molar mass of NaCl = Molar mass of 1 Sodium (Na) atom + Molar mass of 1 Chlorine .Molar Mass: There is one Sodium atom and one Chlorine atom in the formula for Sodium Chloride(NaCl). As a result, the molar mass of sodium chloride will be equal to the sum of the two atoms’ molar masses. Sodium has an atomic mass of 22.99 g/mol, while chlorine has an atomic mass of 35.45 g/mol.
Molar concentration, also known as molarity, and can be denoted by the unit M, molar. To prepare 1 L of 0.5 M sodium chloride solution, then, as per the formula, use 29.22 g of sodium chloride (0.5 mol/L * 1L * 58.44 g/mol = 29.22 g). The mass molarity calculator tool calculates the mass of compound required to achieve a specific molar . Molar mass: 58,44277 g/mol. The number of atoms: 2 - Na, Cl. Melting point: 800.8 ° C. Boiling point: 1465 ° C. Solubility: in water, in methanol, in ammonia. Sodium chloride is the sodium salt of hydrochloric acid. It is included in table salt as the main component. It is present in large quantities in seawater and is a precondition for its .
The molar mass of Chlorine is 35.45 g/mol. [2] Now, to calculate the molar mass of NaCl, you just have to add the molar mass of all the individual atoms that are present in NaCl. You can see that in NaCl, there is 1 Sodium atom and 1 Chlorine atom. So, Molar mass of NaCl = Molar mass of 1 Sodium (Na) atom + Molar mass of 1 Chlorine .
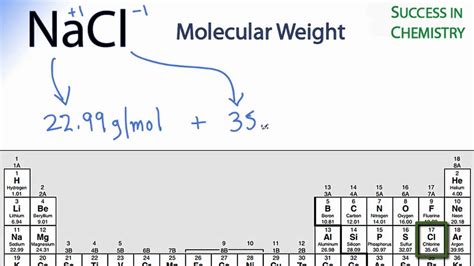
Molar Mass: There is one Sodium atom and one Chlorine atom in the formula for Sodium Chloride(NaCl). As a result, the molar mass of sodium chloride will be equal to the sum of the two atoms’ molar masses. Sodium has an atomic mass of 22.99 g/mol, while chlorine has an atomic mass of 35.45 g/mol.Multiply the number of atoms by the atomic weight of each element found in steps 1 and 2 to get the mass of each element in 2NaCl: Molar Mass (g/mol) Na (Sodium) 2 × 22.98976928 = 45.97953856. Cl (Chlorine) 2 × 35.453 = 70.906. 4. Sum Each Element's Mass. Finally, add together the total mass of each element to get the molar mass of 2NaCl:Multiply the number of atoms by the atomic weight of each element found in steps 1 and 2 to get the mass of each element in (Na)Cl: Molar Mass (g/mol) Na (Sodium) 1 × 22.98976928 = 22.98976928. Cl (Chlorine) 1 × 35.453 = 35.453. 4.Next, using the periodic table, find the atomic mass in g/mol of each element (the molar mass of an element is equal to its atomic mass): Molar Mass (g/mol) Na (Sodium) 22.98976928: Cl (Chlorine) 35.453: 3. Compute Mass of Each Element.

Solutions to Example 6.2.2, Summing the molar masses of the atoms in the NaCl formula unit gives. 1 Na molar mass: 22.99 g. 1 Cl molar mass: 35.45 g. Total: 58.44 g. The mass of 1 mol of NaCl is 58.44 g. Multiplying the molar mass of each atom by the number of atoms of that type in bilirubin’s formula and adding the results, we get. Why Table Salt Isn't Really NaCl . If you had a pure sample of sodium chloride, it would consist of NaCl. However, table salt actually isn't pure sodium chloride. . Molar Mass: 58.44 grams per mole Appearance: Pure sodium chloride forms odorless, colorless crystals. Many small crystals together reflect light back, making the salt appear .molar mass of nacl formula mass of naclThe molar mass of NaCl is 58.44 g / m o l. Was this answer helpful? 6. Click here:point_up_2:to get an answer to your question :writing_hand:what is the molar mass of nacl table salt.Multiply the number of atoms by the atomic weight of each element found in steps 1 and 2 to get the mass of each element in (2NaCl): Molar Mass (g/mol) Na (Sodium) 2 × 22.98976928 = 45.97953856. Cl (Chlorine) 2 × 35.453 = 70.906. 4.
Explanation of how to find the molar mass of NaCl: Sodium chloride.A few things to consider when finding the molar mass for NaCl:- make sure you have the cor.
Ao se registrar, você pode receber até R$8888 de bônus de boas-vindas, um dos maiores do mercado brasileiro de cassinos online. Descubra o 777bet Cassino, onde a .
molar mass of nacl|formula mass of nacl